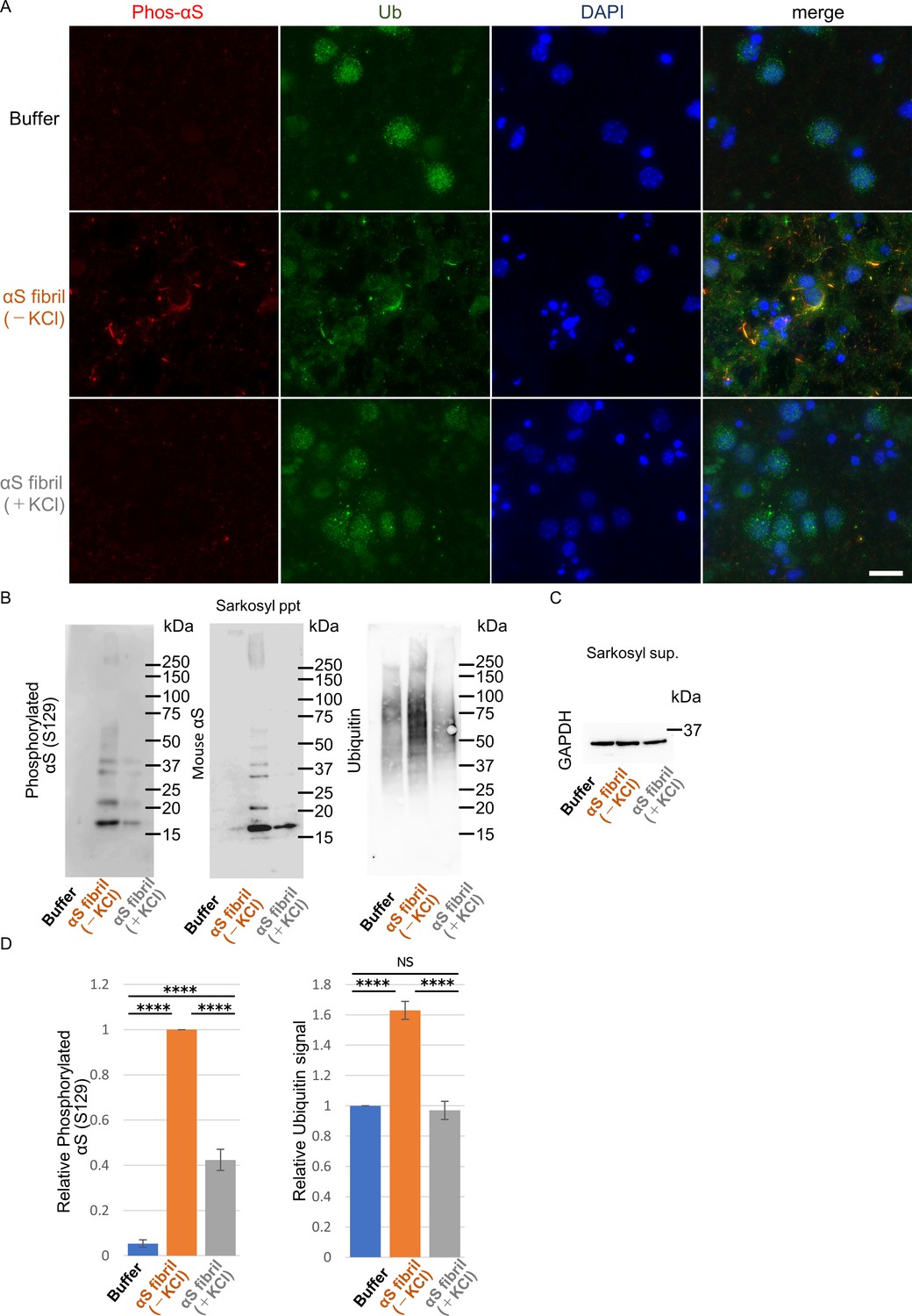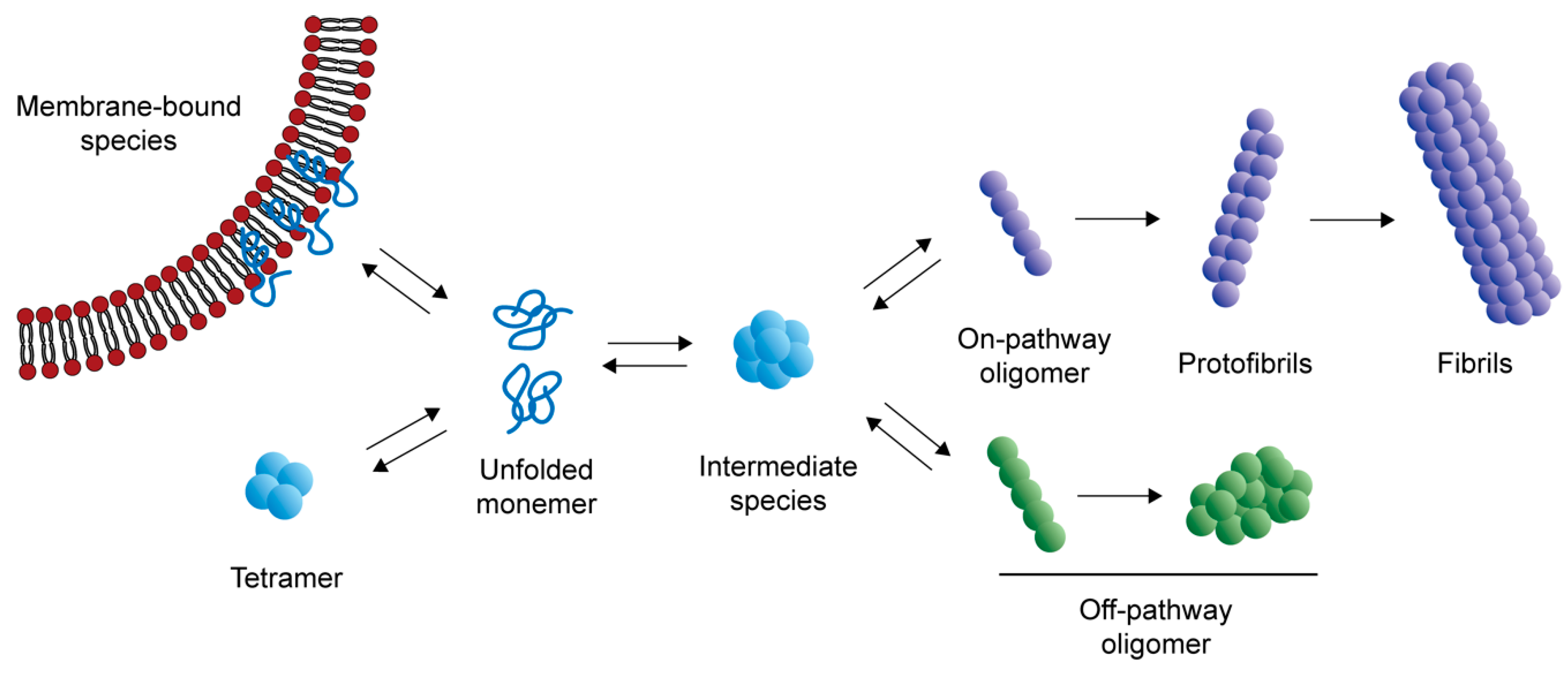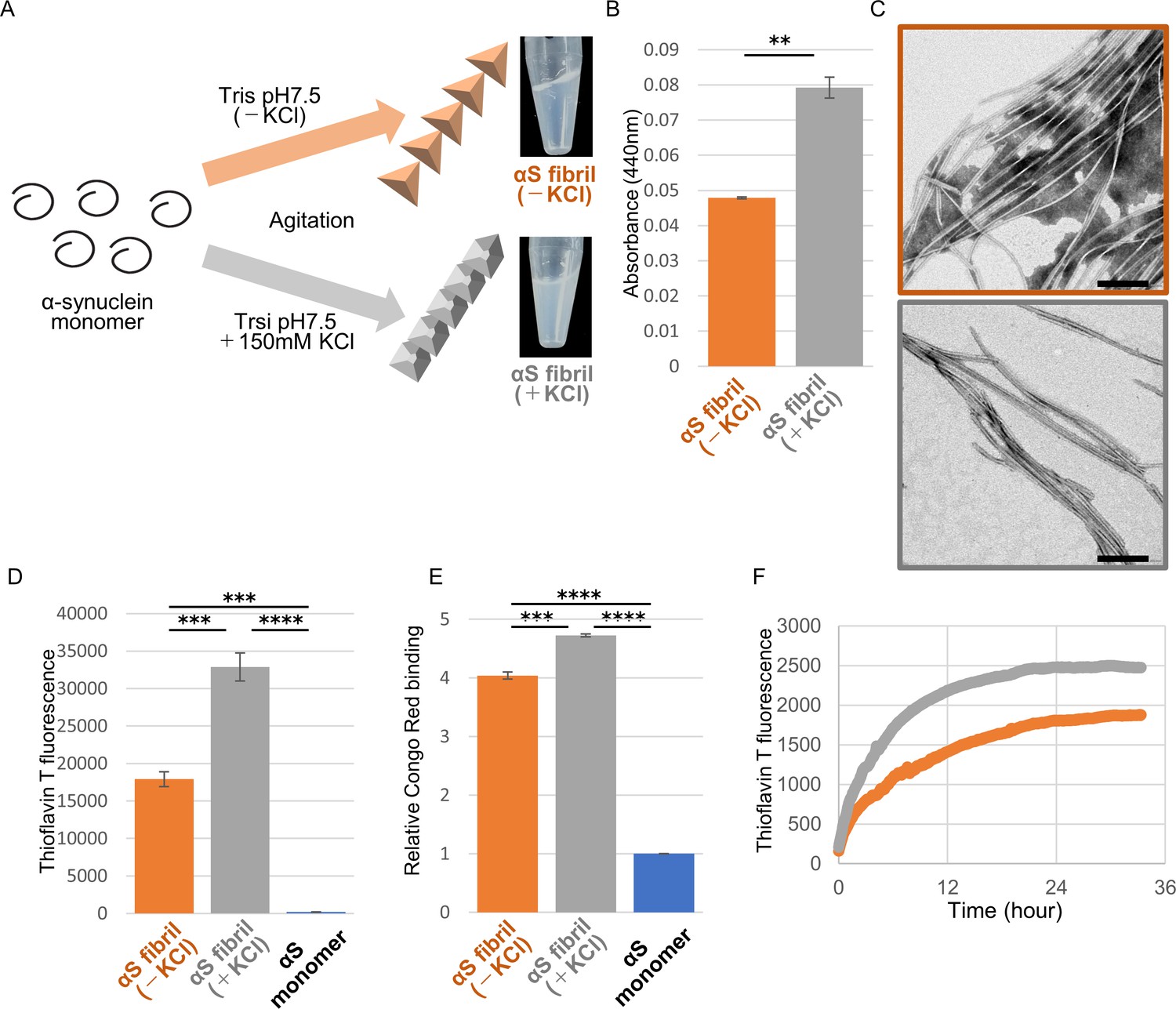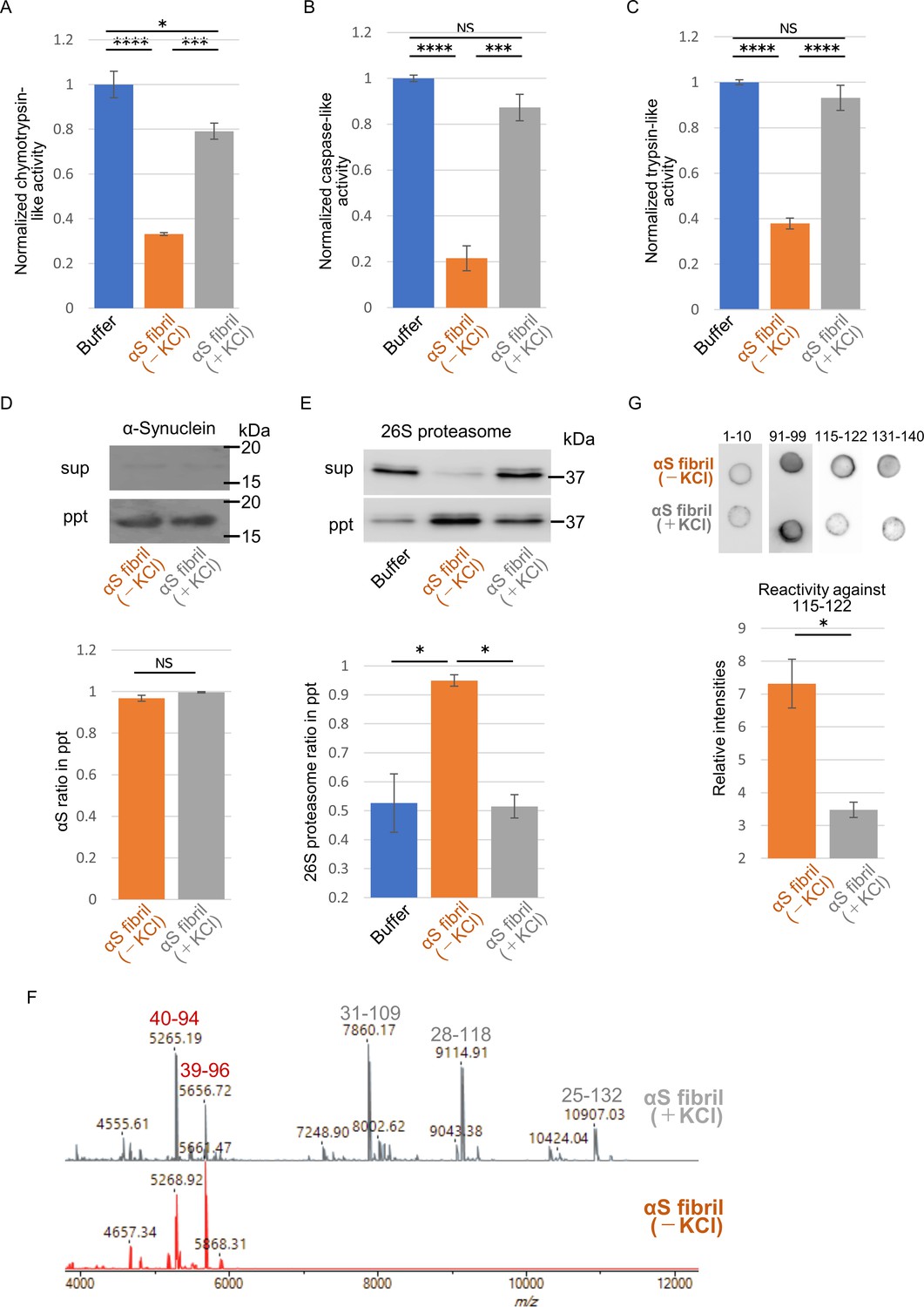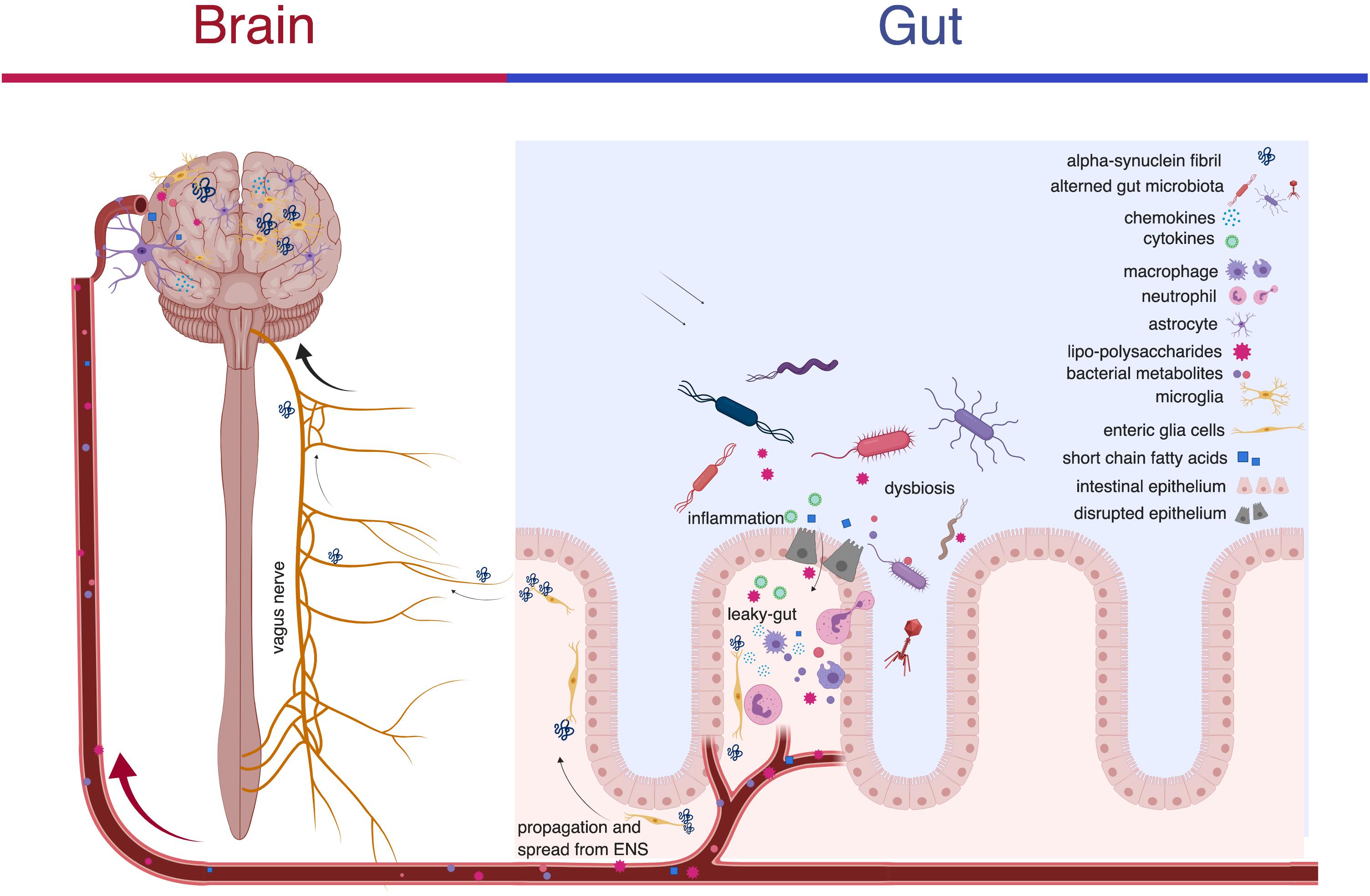
Frontiers | Alpha-Synuclein Pathology and the Role of the Microbiota in Parkinson's Disease | Neuroscience

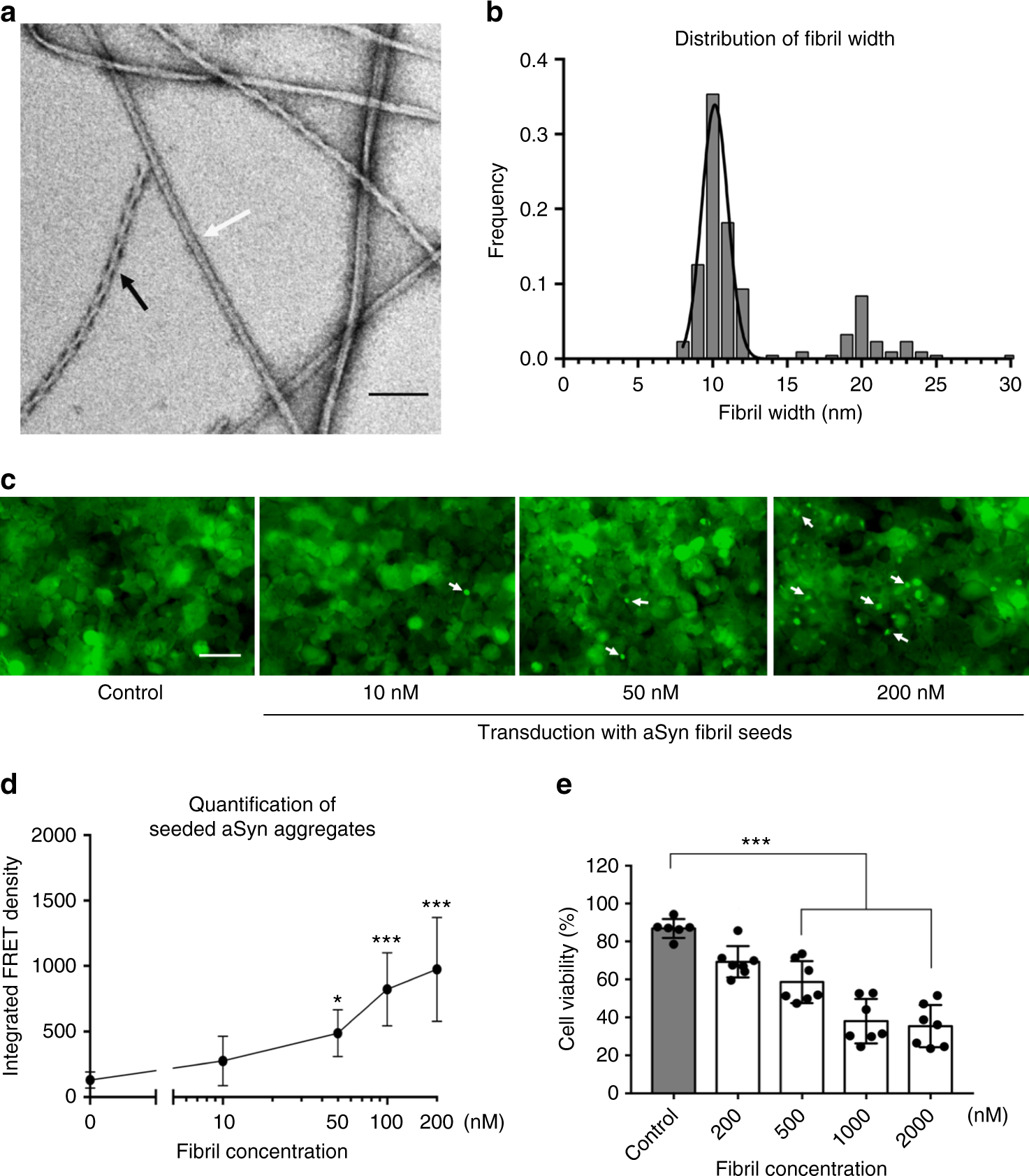
Alpha-synuclein stepwise aggregation reveals features of an early onset mutation in Parkinson's disease | Communications Biology

Sorting out release, uptake and processing of alpha‐synuclein during prion‐like spread of pathology - Tyson - 2016 - Journal of Neurochemistry - Wiley Online Library
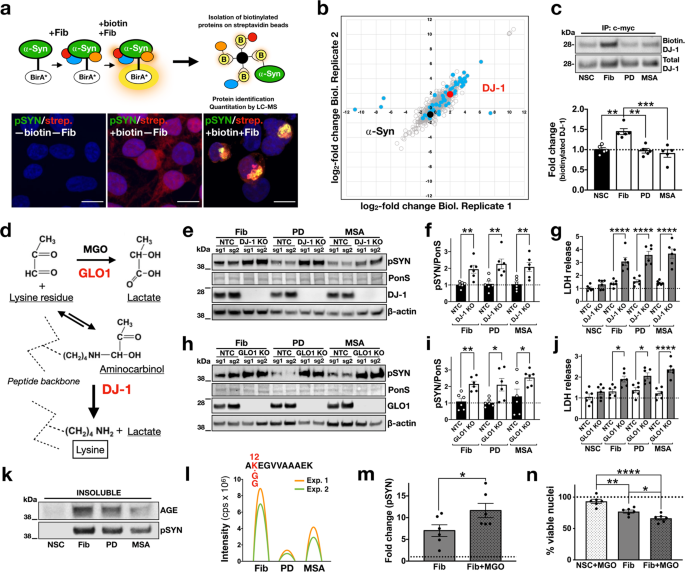
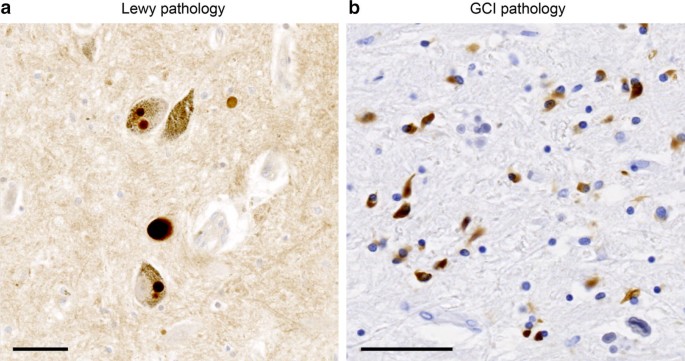
Phenotypic manifestation of α-synuclein strains derived from Parkinson's disease and multiple system atrophy in human dopaminergic neurons | Nature Communications
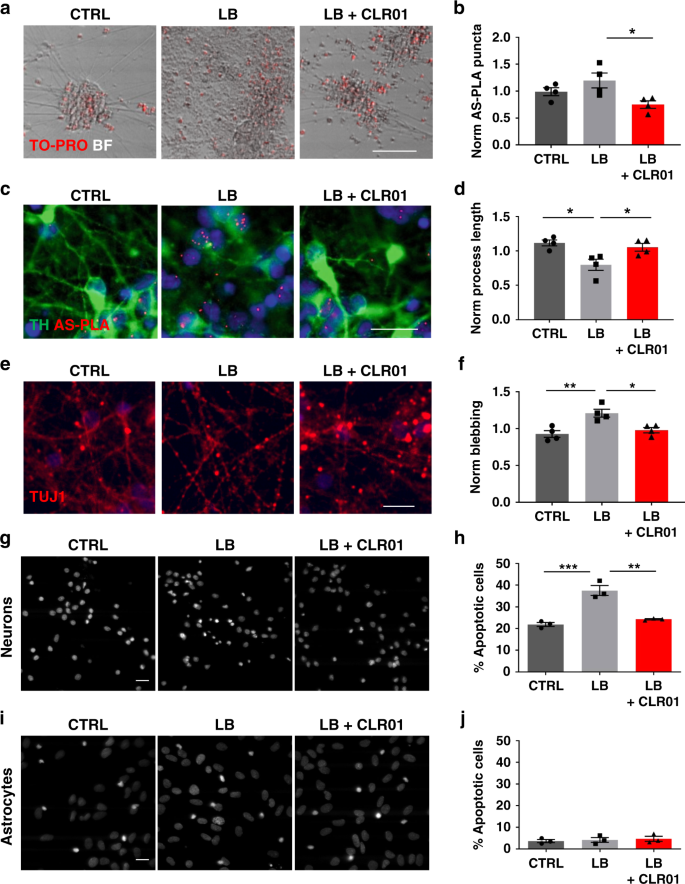
CLR01 protects dopaminergic neurons in vitro and in mouse models of Parkinson's disease | Nature Communications

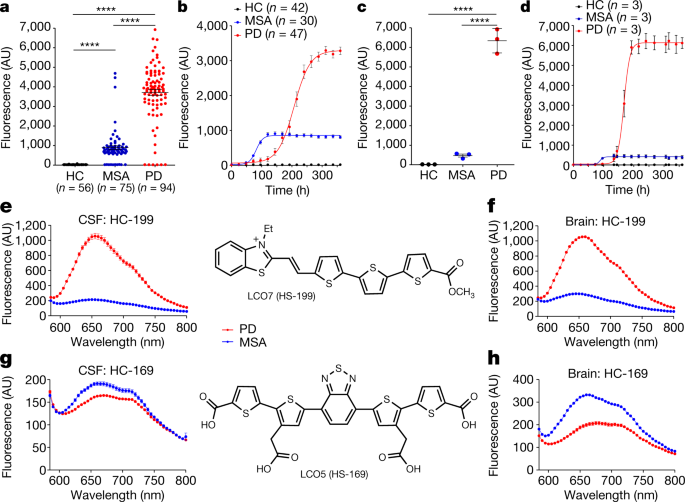
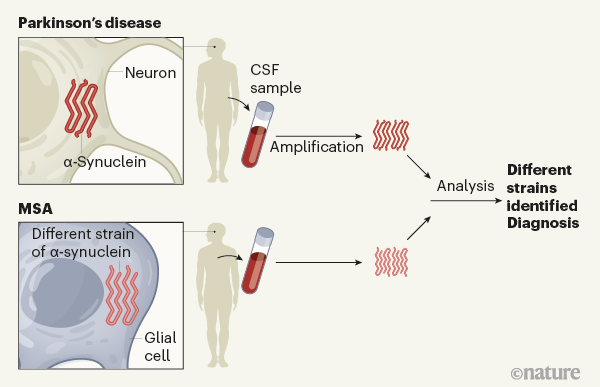
Parkinson's disease and multiple system atrophy have distinct α-synuclein seed characteristics - Journal of Biological Chemistry
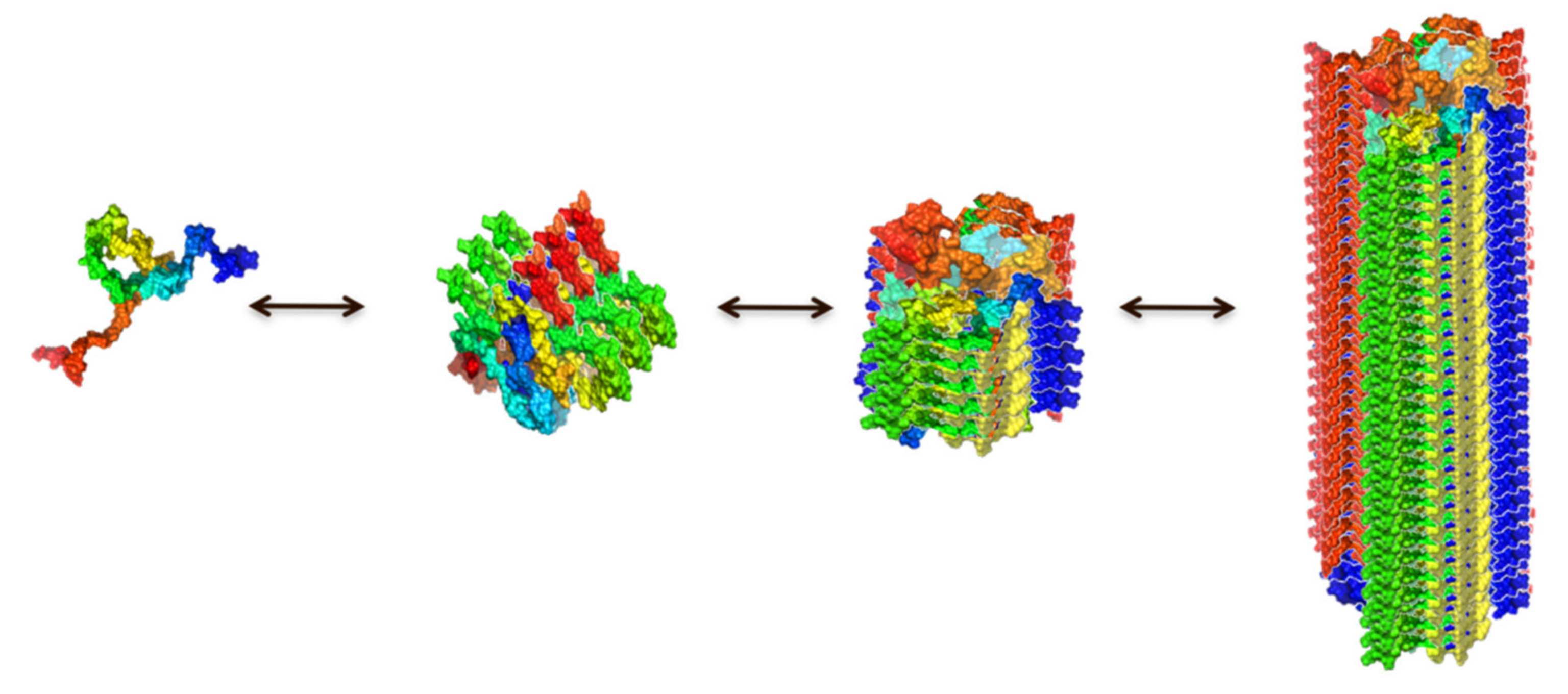
IJMS | Free Full-Text | Multiplicity of α-Synuclein Aggregated Species and Their Possible Roles in Disease | HTML
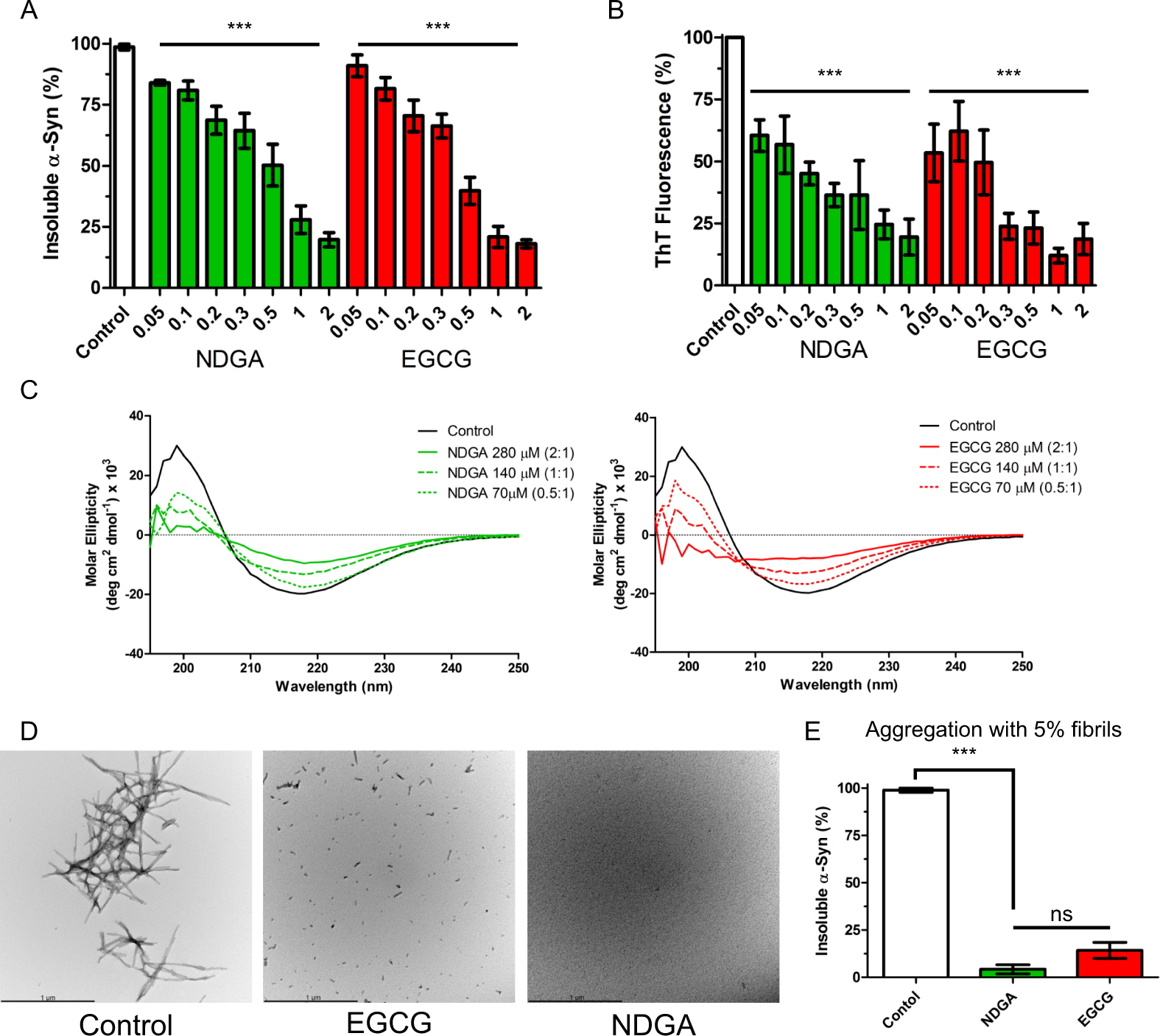
Cyclized NDGA modifies dynamic α-synuclein monomers preventing aggregation and toxicity | Scientific Reports
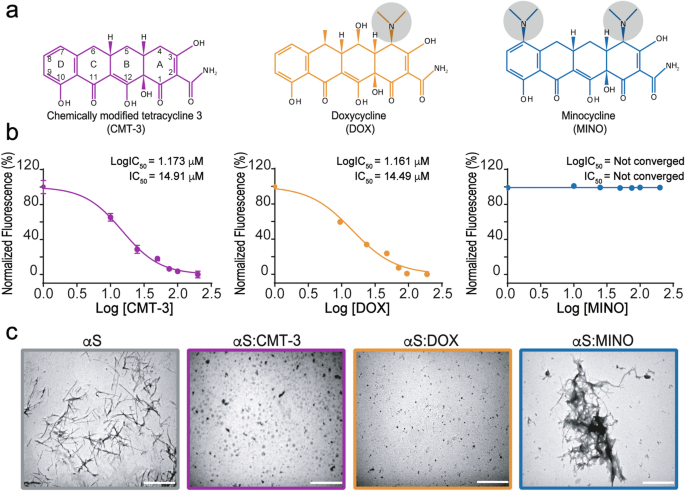
CMT-3 targets different α-synuclein aggregates mitigating their toxic and inflammogenic effects | Scientific Reports

Anti-amyloid Compounds Inhibit α-Synuclein Aggregation Induced by Protein Misfolding Cyclic Amplification (PMCA)* - Journal of Biological Chemistry

Navigating the dynamic landscape of alpha-synuclein morphology: a review of the physiologically relevant tetrameric conformation Lucas HR, Fernández RD - Neural Regen Res

A natural product inhibits the initiation of α-synuclein aggregation and suppresses its toxicity | PNAS

Distinct α-Synuclein strains and implications for heterogeneity among α-Synucleinopathies - ScienceDirect
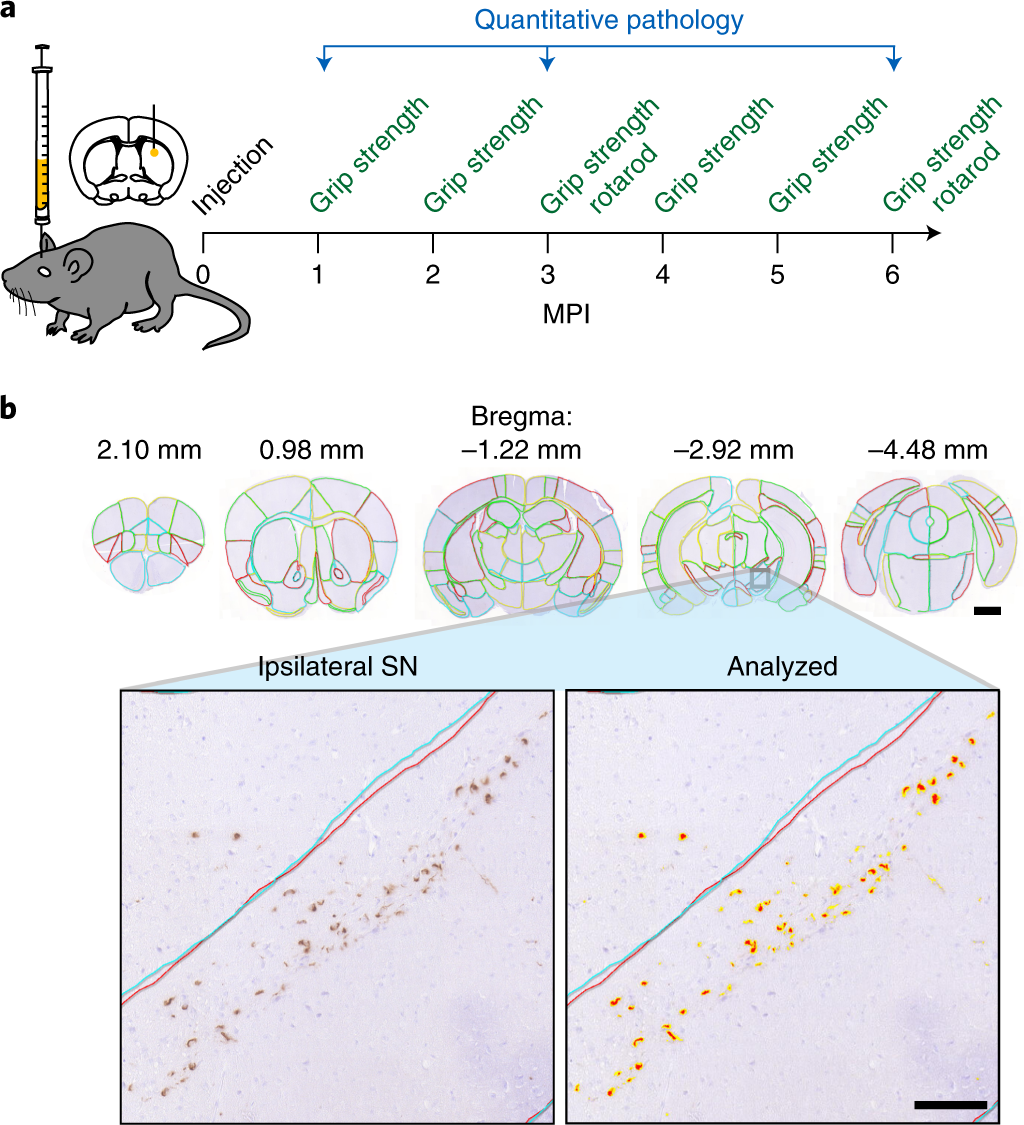
Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis | Nature Neuroscience